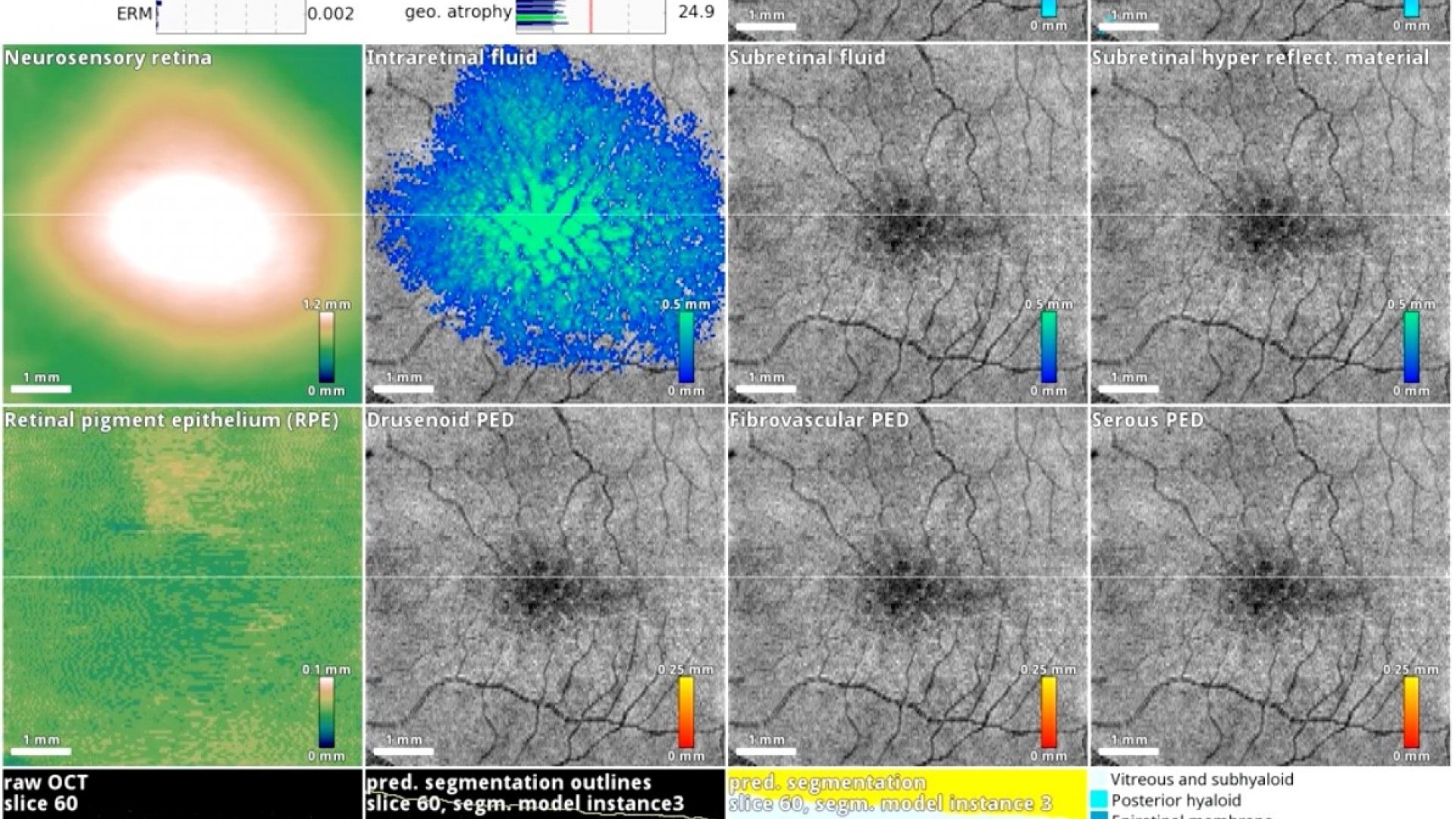
Optical coherence tomography (OCT), first described in 1991, is a form of medical imaging analogous to ultrasonography but which measures the reflections of light waves from tissue rather than the echoes of sound.1 In doing so, it generates high-resolution cross-sectional images of tissue in a quick and non-invasive manner, and has become a standard of care for retinal diseases and glaucoma. While predominantly still used in hospital settings, OCT imaging is increasingly widespread in community eye care, and prototype OCT systems are being developed for use by patients themselves in the home.2 Binocular OCT systems are also in development, offering the possibility of automated, comprehensive eye examinations.3
The AI system in action on a patient with diabetic eye disease.
With such large volumes of data being generated in several settings, retinal OCT imaging is particularly amenable to analysis using deep learning.4 Much of this initial work has focused on automated, quantitative evaluation of OCT images.5 These systems delineate or “segment” morphologic compartments of interest (e.g. vitreous, retina and choroid), retinal sublayers, or disease features (e.g. intraretinal fluid), and then generate tissue volumes and/or thickness maps. These measurements can then be used in clinical practice to guide treatment, in clinical trials as surrogate endpoints, and in exploratory research as prognostic indicators. More recent work has focused on the clinical classification of OCT scans for the purposes of retinal disease triage and to assist in the diagnosis of glaucomatous optic neuropathy.6,7 A number of these systems are in commercial development and seem likely to be in widespread use in the coming years.
New applications for retinal OCT are rapidly emerging. Using deep learning, it is possible to predict, in a clinically actionable time frame, the future progression of diseases such as age-related macular degeneration.8 Cross-modality training of deep learning systems has also shown promise for improving the diagnostic accuracy of simple imaging modalities using labels from a more advanced modality (e.g., using OCT-derived gold standard labels for training of deep learning models on fundus photographic images in diabetic macular oedema and glaucoma).9,10
- Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991; 254(5035): 1178-81.
- Maloca P, Hasler PW, Barthelmes D, et al. Safety and Feasibility of a Novel Sparse Optical Coherence Tomography Device for Patient-Delivered Retina Home Monitoring. Transl Vis Sci Technol 2018; 7(4): 8.
- Walsh AC. Binocular optical coherence tomography. Ophthalmic Surg Lasers Imaging 2011; 42 Suppl: S95-S105.
- Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol 2019; 103(2): 167-75.
- Ting DSW, Peng L, Varadarajan AV, et al. Deep learning in ophthalmology: The technical and clinical considerations. Prog Retin Eye Res 2019; 72: 100759.
- De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med 2018; 24(9): 1342-50.
- Liu H, Li L, Wormstone IM, et al. Development and Validation of a Deep Learning System to Detect Glaucomatous Optic Neuropathy Using Fundus Photographs. JAMA Ophthalmol 2019.
- Yim J, Chopra R, Spitz T, et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat Med 2020; 26(6): 892-9.
- Varadarajan AV, Bavishi P, Ruamviboonsuk P, et al. Predicting optical coherence tomography-derived diabetic macular edema grades from fundus photographs using deep learning. Nat Commun 2020; 11(1): 130.
- Medeiros FA, Jammal AA, Thompson AC. From Machine to Machine: An OCT-Trained Deep Learning Algorithm for Objective Quantification of Glaucomatous Damage in Fundus Photographs. Ophthalmology 2019; 126(4): 513-21.